A replacement reaction occurs when elements switch places in compounds. A BC B AC.

Single Replacement Single Displacement Reaction
A double replacement reaction occurs when two ionic compounds exchange ions producing two new ionic compounds.

. A single replacement reaction occurs when one element replaces another in a single compound. This can either be in the form of a single replacement reaction or a double replacement reaction. This type of reaction involves ions.
Only a more reactive element can replace the other element in the compound with which it reacts. Single replacement reactions take place because some elements are more reactive than other ele. WHY do single replacement reactions occur.
ABC B AC. This type of reaction has the general equation. ZnCuCl2 Cu ZnCl2.
When a replacement reaction occurs a new aqueous compound and a different pure element will be generated as products. Uh they do occur. A halogen replaces another halogen that is in solution.
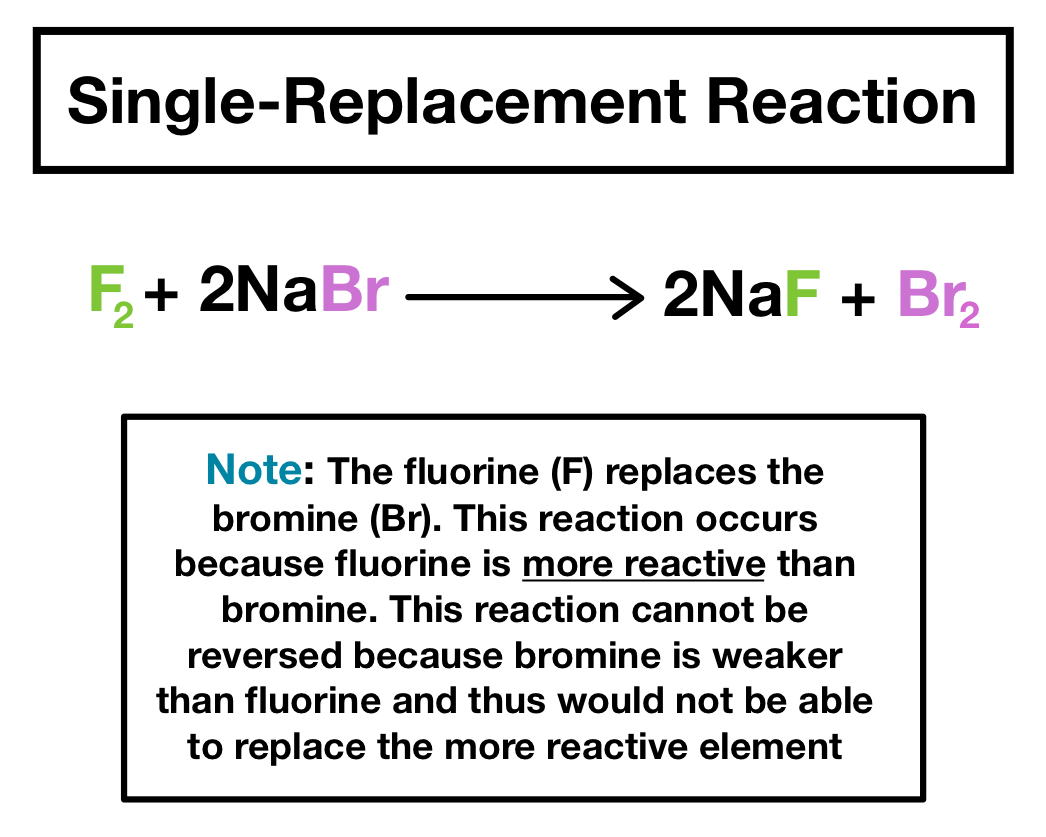
Because fluorine is above bromine on the periodic table a singlereplacement reaction will occur and the products of the reaction will be CaF 2 and Br 2. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound. When atoms and molecules come together and split chemical reactions occur.
A single replacement reaction is a chemical reaction where one element replaces another in a compound. A metal only replaces a metal and a nonmetal only replaces a nonmetal. A metal only replaces a metal and a nonmetal only replaces a nonmetal.
Generally more reactive elements replace less reactive elements. This type of reaction involves ions. Single replacement reactions occur when A is more reactive than B or product AC is more stable than BC.
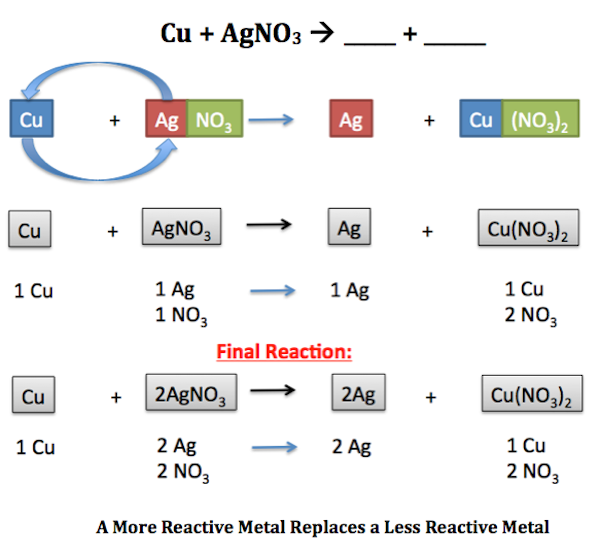
The typical demonstration of this in high school chemistry classes everywhere is to suspend a piece of copper wire in a solution of. There are two types of single replacement reactions. Because iodine is below chlorine on the periodic table a singlereplacement reaction will not occur.
Note that if both of the predicted products are soluble a precipitation reaction will not occur. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound. A single-displacement reaction occurs when an element replaces another element in a compound.
When an element replaces another element from a compound a single displacement reaction has occurred a more active element with replace a less active element from its compound. A BC B AC. The general pattern of a single replacement reaction is shown below.
Many single-replacement reactions occur in an aqueous solution. A BC B AC. In double replacement reactions the elements get replaced in both the reacting compounds.
A single replacement reaction occurs when one element replaces another element in. Generally more reactive elements replace less reactive elements. It is also known as a single displacement reaction.
Why do some double displacement reactions not occur. A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound. A metal replaces another metal that is in solution.
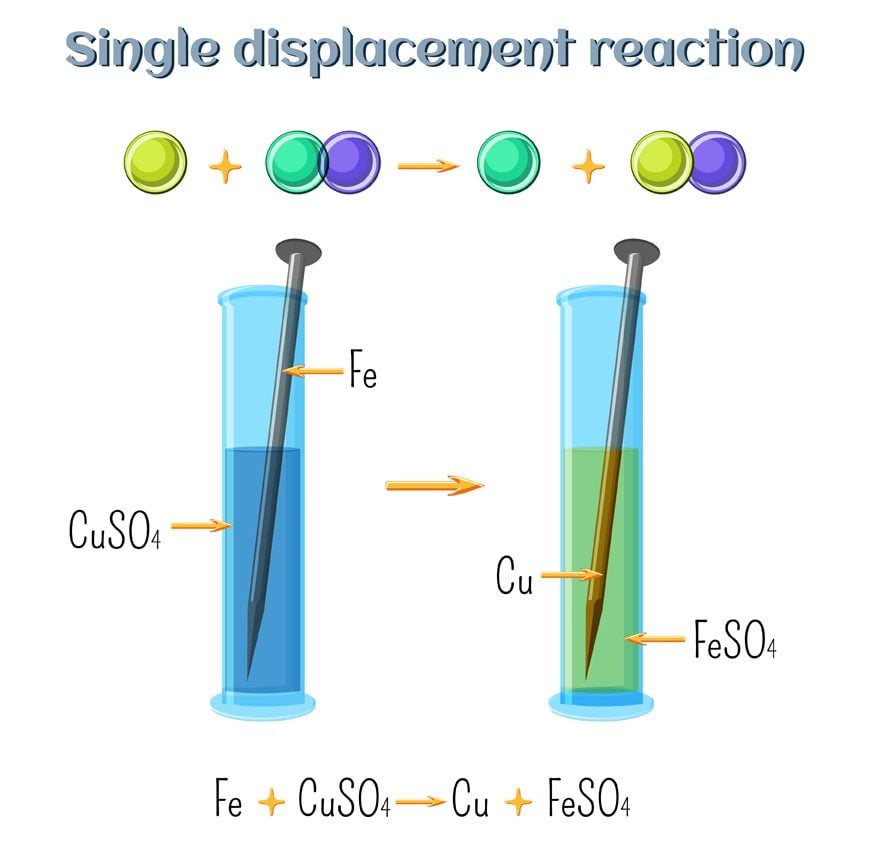
The general form of a single replacement reaction chemical equation is. Zn CuCl_2 Cu ZnCl_2 A halogen replaces another halogen that is in solution. Only a more reactive element.
A single-displacement reaction occurs when an element replaces another element in a compound. A metal replaces another metal that is in solution. A single replacement reaction occurs when one element replaces another element in.
A BC B AC Example. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. You may ask Why do double replacement reactions occur.
When a double displacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state. A single replacement reaction occurs when one element replaces another element in one compound. View the full answer.
There are two types of single replacement reactions. This type of reaction is represented by. A replacement reaction occurs when elements switch places in compounds.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable. An example of a single replacement reaction occurs when potassium K reacts with water H2O.
Single Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Single Replacement Reaction Definition And Examples
Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Single Replacement Reactions And Net Ionic Equations Youtube

Single Replacement Single Displacement Reaction
Single Replacement Reaction Vs Double Replacement Reaction Differences Examples
0 comments
Post a Comment